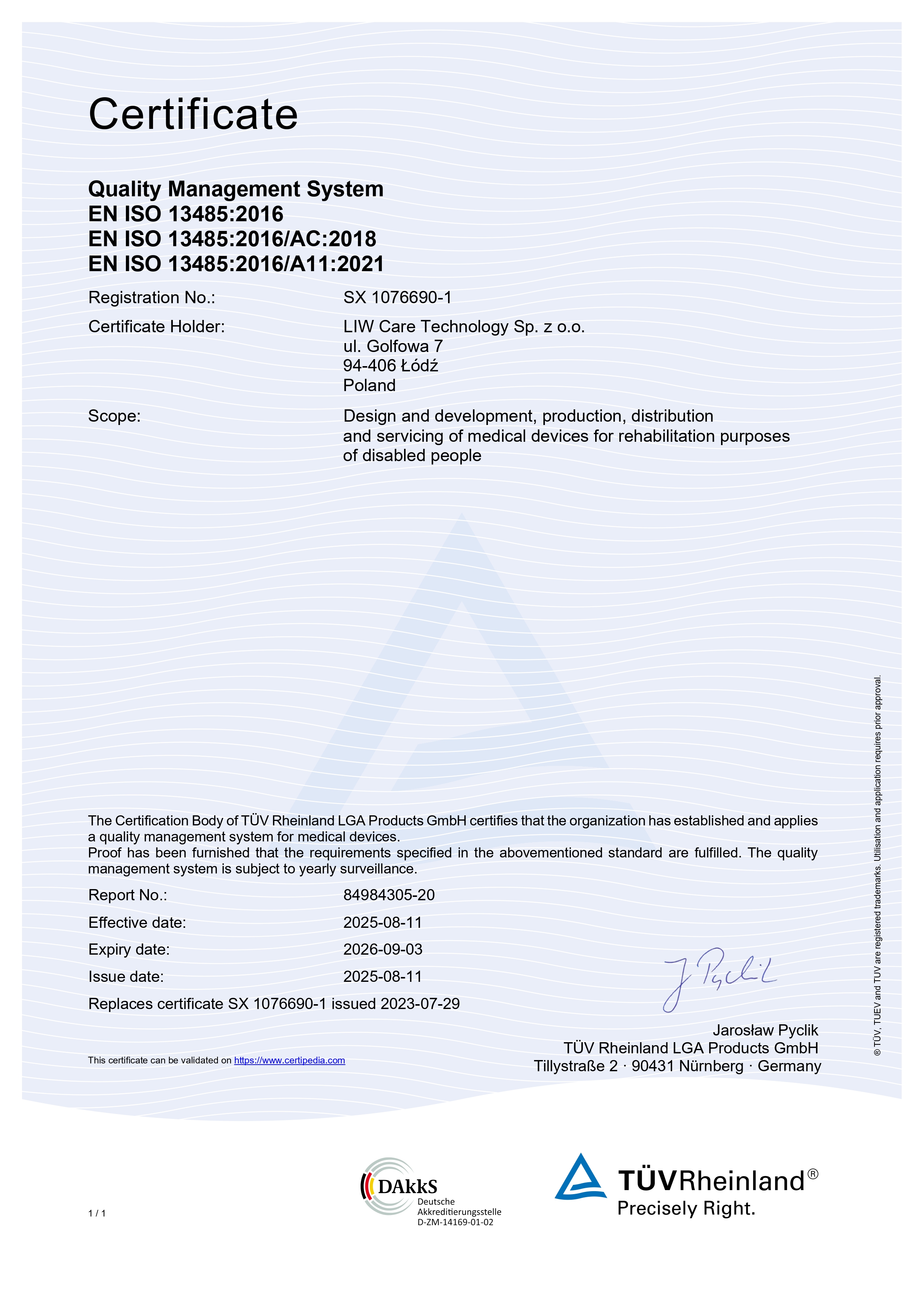
We have implemented and keeping the quality management system complies with the requires of the EN ISO 13485:2016 standard. Conformity of the quality management system is confirmed with certificate granted by TÜV Rheinland Polska Sp. z o.o. – which concerns design and development, manufacturing, distribution and service of medical devices used for rehabilitation of disabled people.
Under the quality management system:
- processes and their use necessary in the quality management system in LIW CARE TECHNOLOGY has been identified,
- we have identified the sequence of these processes and their interactions,
- we have defined the criteria and methods needed to ensure the effectiveness of both the process and the supervision of these processes,
- we have provided the resources and information availability which is necessary to support the process and its monitoring,
- we have monitored, measured and analysed processes,
- we have provided an approach to the control of the relevant processes required for a quality management system based on risk,
- we have implemented the necessary actions to achieve the planned results and maintain the effectiveness of these processes.
The implemented quality management system for medical devices, in addition to the transparency of internal processes, ensures compliance with international law, enhances the safety of the offered equipment and affects the efficiency of the entire company.
Manufactured medical devices meet the requirements of the applicable regulations, including Regulation (EU) 2017/745 of the European Parliament and of the Council.
Any medical device has an EC declaration of conformity and a CE-labeled plate. Medical devices are reported and registered in the Polish Office for Registration of Medicinal Products, Medical Devices and Biocidal Products.
We undertake to comply with the requirements of the applicable regulations and to continuously improve the effectiveness of the implemented quality management system.

 Polski
Polski  English
English 





